Intended for US Healthcare Professionals only.
The FIRST FDA-Approved and ACIP-Recommended Mpox Vaccine1,2

AGAINST MPOX WITH JYNNEOS*
*Vaccination with JYNNEOS may not protect all recipients.
ABOUT MPOX*
Mpox is a vaccine-preventable, viral disease that can cause symptoms such as a painful rash, fever, and swollen lymph nodes and can result in serious complications.3
There are 2 distinct clades4:
Clade
I
Found mainly in
Central Africa
Clade
II
Clade IIa found mainly in West Africa; Clade IIb found worldwide
Symptoms, complications, and mortality vary between the different clades.4,5
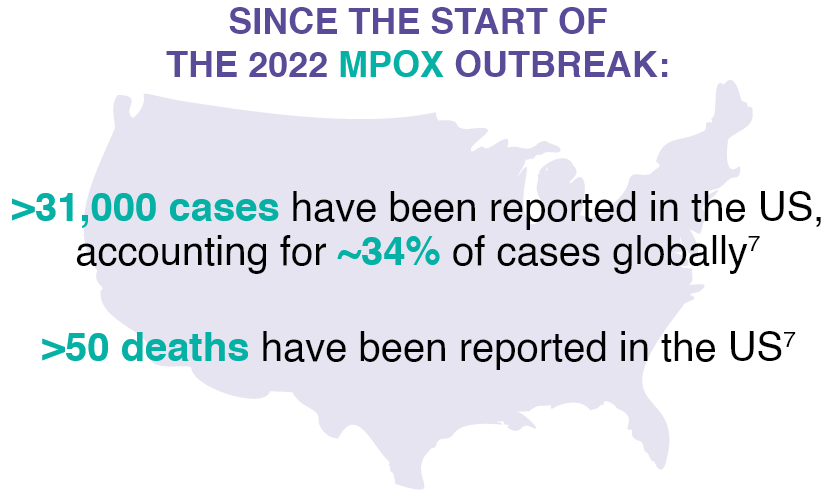
THERE WAS AN UNPRECEDENTED GLOBAL MPOX OUTBREAK IN 2022 (CAUSED BY
CLADE IIb STRAIN)3 — AND IT HASN’T GONE AWAY6†
- Most common presentation: systemic illness, including fever and myalgia5
- Characteristic rash, with papules that evolve to vesicles, pustules, and crusts in the genital, anal, or oral regions, and often involve the mucosa5
- Infections with Clade IIb are rarely fatal (>99% of people who get this form of the disease are likely to survive)8
- Up to 40% of cases had complications requiring medical treatment, and 1-13% required hospital admission for treatment or isolation5
Anyone can be infected by the mpox virus3; however, since 2022, most cases in the US have been among at-risk groups7,9
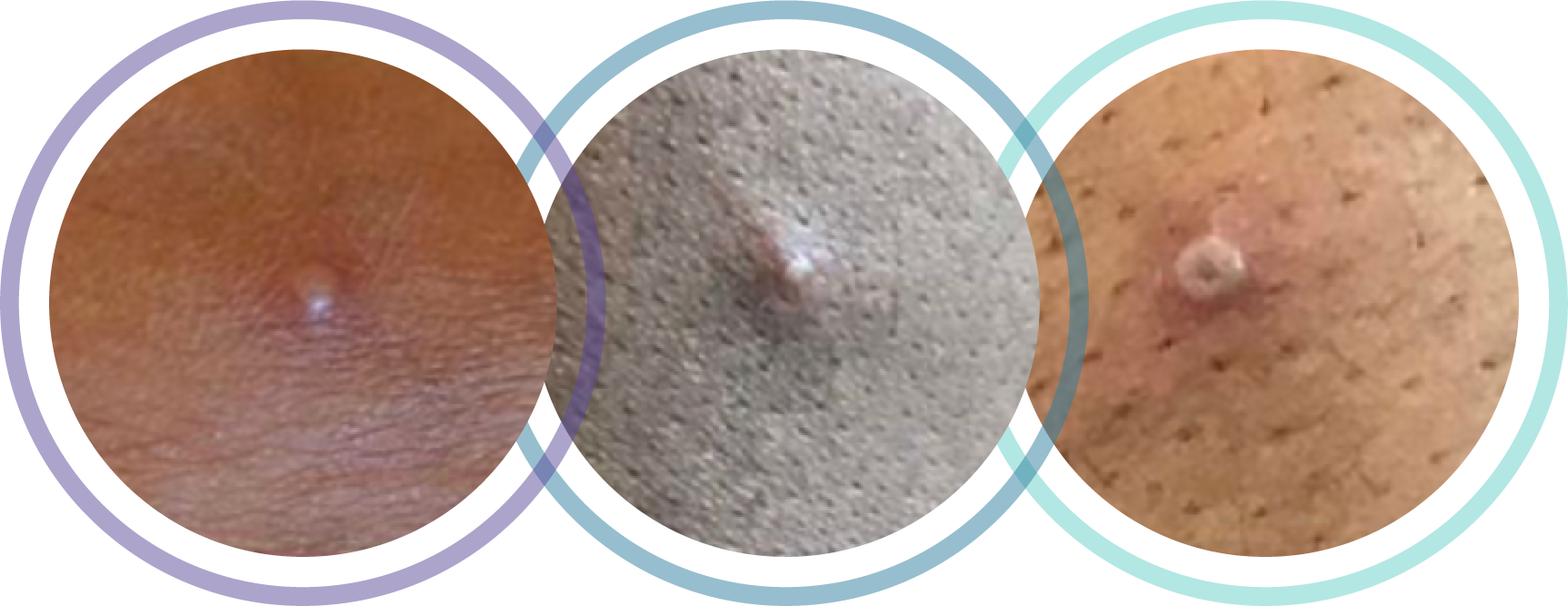
Photo credit: UK Health Security Agency10
The ACIP recommends mpox vaccination for all at-risk individuals aged
≥18 years old.2
Persons at risk include2:
- Gay, bisexual, and other men who have sex with men, transgender or nonbinary people who in the past 6 months have had one of the following:
- A new diagnosis of ≥1 sexually transmitted diseases
- More than one sexual partner
- Sex at a commercial sex venue
- Sex in association with a large public event in a geographic area where mpox transmission is occurring
- Sexual partners of persons with the risks described in above
- Persons who anticipate experiencing any of the above
Most at-risk people are still not fully vaccinated against mpox
There are ~2 million at-risk individuals eligible for mpox vaccination in the US11
As of January 2024, more than half of at-risk individuals are completely unvaccinated12‡
The risk for future mpox outbreaks in the US remains.13 Don’t wait for another surge.
If your patient is at risk for mpox, consider vaccination.
Click to read the full ACIP recommendation and see the most recent updates.
*The CDC, HHS, and WHO adopted “mpox” as the term used to refer to monkeypox disease on November 28, 2022.14
†The mpox outbreak continues in most WHO regions, at a low level of transmission in the Western Pacific and South-East Asia, while more extensive transmission is observed in the European Region and in the Region of the Americas (as of December 22, 2023).6
‡US mpox vaccine administration data no longer being tracked as of January 10, 2024.12
Effectiveness Data
Vaccine effectiveness was inferred from clinical immunogenicity data and supported by data from animal challenge studies.15
Study 7 – a randomized, open-label study conducted at US military facilities in South Korea15
Study design: Healthy, smallpox vaccine-naive adults aged 18-42 years (N=433) received either 2 doses of JYNNEOS (n=220) administered 28 days apart or 1 dose of ACAM2000® (Smallpox [Vaccinia] Vaccine, Live) (n=213).
Primary endpoint: Geometric mean titer (GMT) of vaccinia-neutralizing antibodies assessed by Plaque Reduction Neutralization Test (PRNT) at “peak visits” defined as 2 weeks after second dose of JYNNEOS and 4 weeks after single dose of ACAM2000.
Peak visit neutralizing antibody responses induced by JYNNEOS were non-inferior to those elicited by ACAM2000.
Comparison of Vaccinia-Neutralizing Antibody Responses Following Vaccination with JYNNEOS or ACAM2000 in Healthy Smallpox Vaccine-Naïve Adults 18 Through 42 Years of Age, Study 7,
Per Protocol Set for Immunogenicity
*Non-inferiority of the “peak visit” PRNT GMT for JYNNEOS compared to ACAM2000 was demonstrated as the lower bound of the 1-sided 97.5% CI for the GMT ratio (JYNNEOS/ACAM2000) was >0.5.
Animal Challenge Studies
Efficacy of JYNNEOS to help protect cynomolgus monkeys (Macaca fascicularis) against a mpox virus challenge was evaluated in several studies15
Animals were given either placebo or JYNNEOS subcutaneously on Days 0 and 28. Animals were challenged with MPXV on Day 63.

Across all studies, 80-100% of JYNNEOS-vaccinated animals survived compared to 0-40% of control animals.
Real-world Effectiveness
Data from 3 case-control studies suggest the vaccine effectiveness of JYNNEOS against mpox ranges from 66-89% for full (2 doses) vaccination.16,17
- CDC Epic Cosmos study: US patient records from Cosmos, an electronic health record platform
- CDC multi-jurisdictional study: 12 US jurisdictions’ probable and confirmed mpox case lists
- New York State study: case surveillance data and New York State’s (excluding New York City) immunization registry
†Vaccine effectiveness in Epic Cosmos case-control study adjusted for age, race/ethnicity, social vulnerability index, and immunocompromising conditions. Cases/controls matched on week of index event, HHS region, gender identity. Vaccine effectiveness in multi-jurisdictional case-control study adjusted for age, race/ethnicity, immunocompromised status, reported close contact with a confirmed/suspected mpox case in 3 weeks prior to index event. In the New York State case-control study, conditional logistic regression model was adjusted for diagnosis week, race, age, region within state.
LIMITATIONS: These case-control studies included individuals vaccinated via subcutaneous, intradermal, and heterologous routes of administration.‡ The studies were at risk for selection bias, confounding bias, and misclassification bias. Additional studies are needed.
‡Approved route of administration for JYNNEOS is subcutaneous. Emergency Use Authorization for intradermal administration was issued by FDA on August 9, 2022.1
ADVERSE REACTIONS
The overall clinical trial program included 22 studies and a total of 7,859 individuals aged 18-80 years who received at least 1 dose of JYNNEOS (7,093 smallpox vaccine-naïve and 766 smallpox vaccine-experienced individuals).15
Adverse Reactions – Study 1
Study 1 was a randomized, double-blind, placebo-controlled study conducted in the US in which vaccinia-naïve adults ages 18 to 40 years received either 2 doses of JYNNEOS (N=3003), or 2 injections of Tris-Buffered Saline (placebo, N=1002) 4 weeks apart.
Percentages of Subjects with Solicited Local Injection Site Reactions and Systemic Adverse Reactions within 8 Days of Administration of Any Dose of JYNNEOS in Adults 18 to 40 Years of Age, Study 1
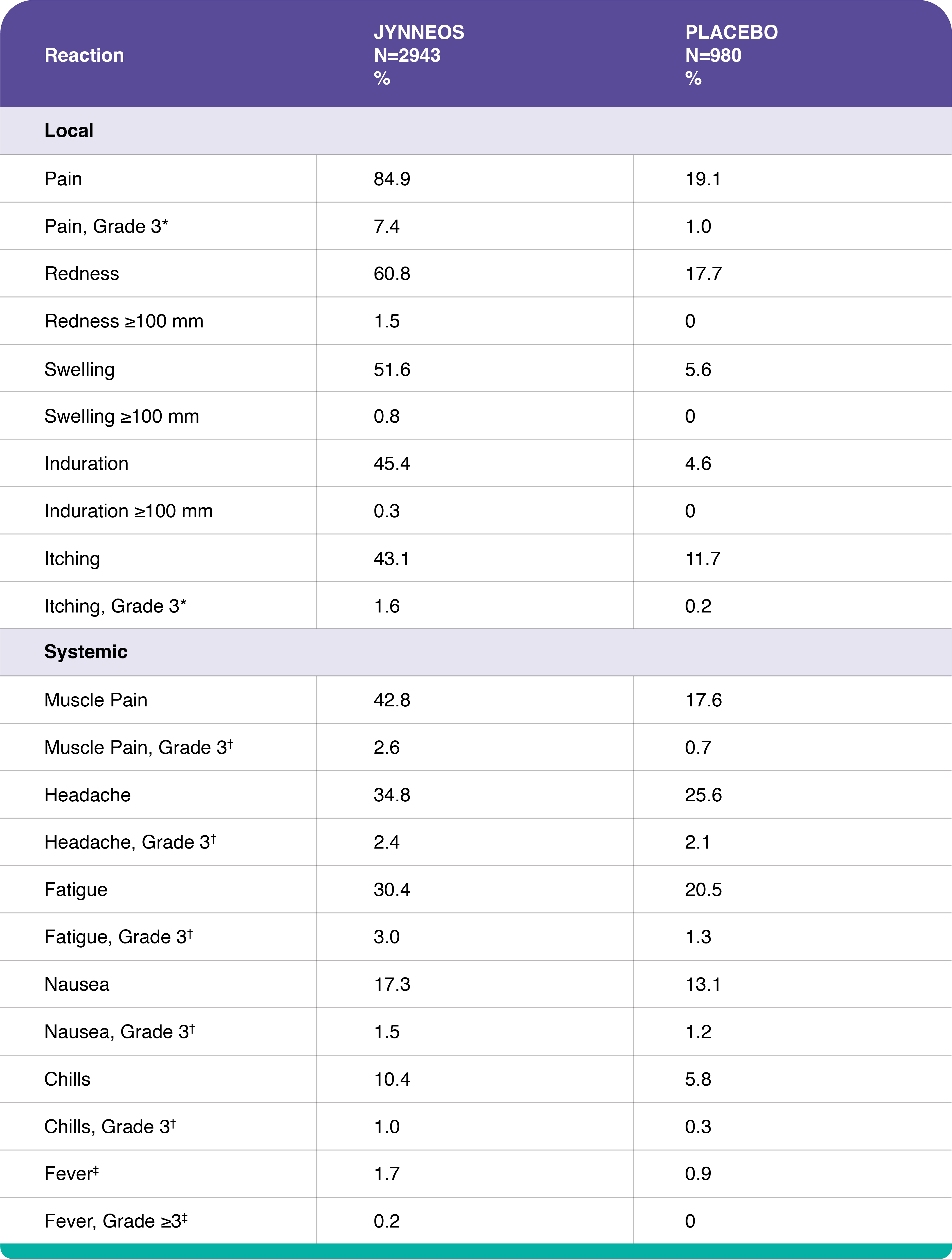
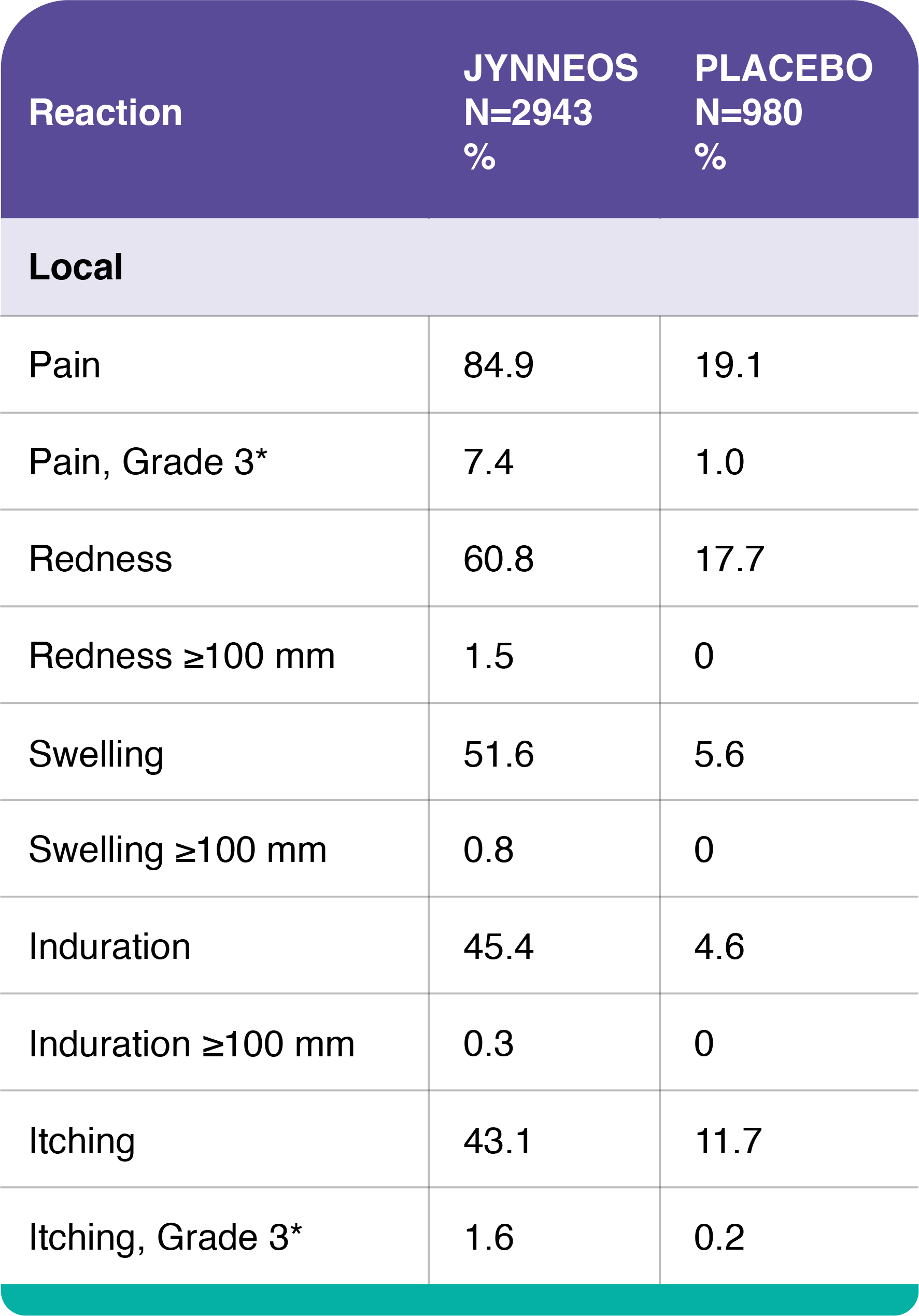
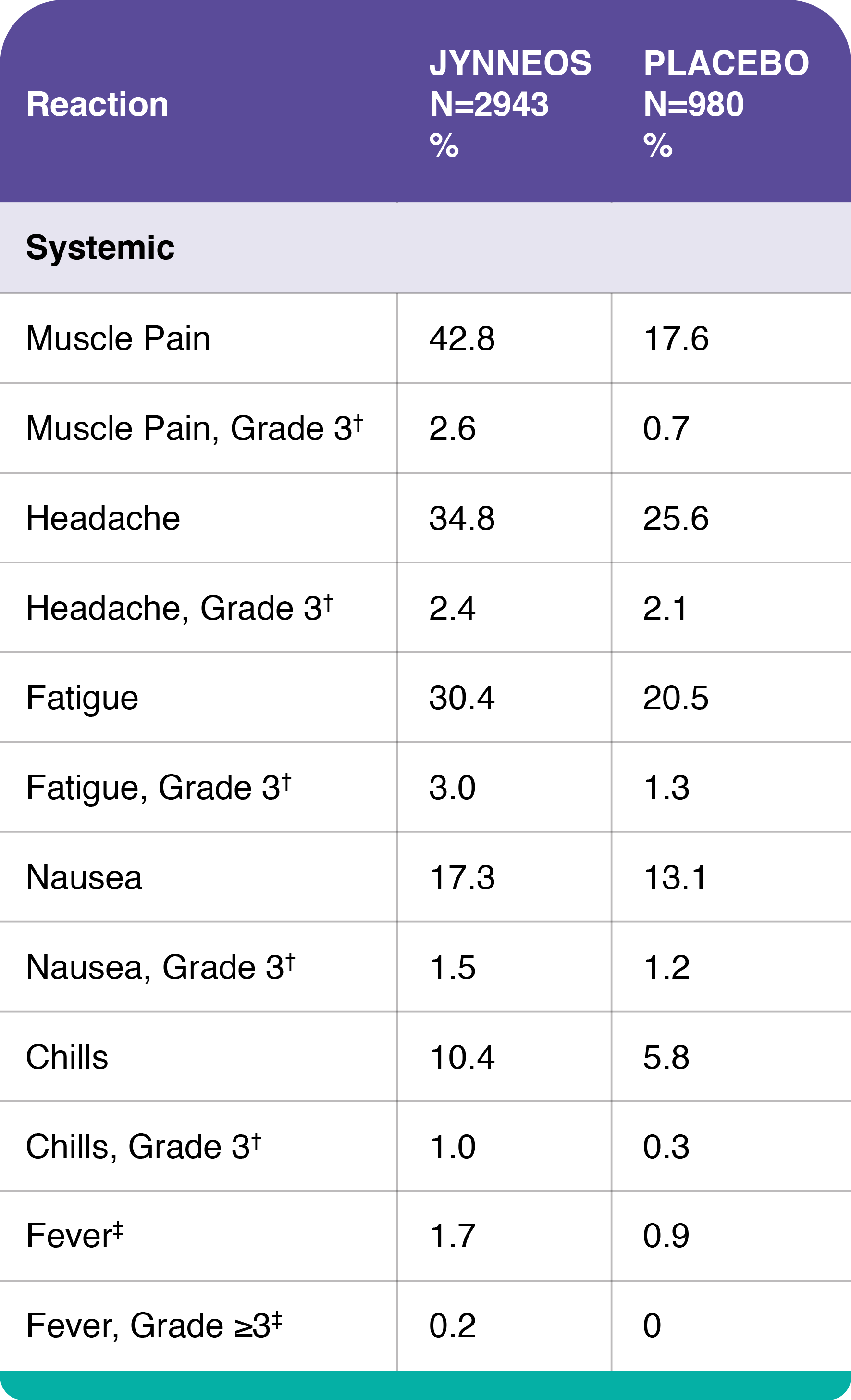
*Grade 3 pain defined as spontaneously painful.
†Grade 3 itching, muscle pain, headache, fatigue, nausea, and chills defined as preventing routine daily activities.
‡Fever defined as oral temperature ≥100.4°F (≥38°C), Grade ≥3 fever defined as ≥102.2°F (≥39.0°C).
Real-world Safety data
Data collected from multiple CDC vaccine safety monitoring systems (90% of VAERS reports were submitted in 2022) have identified no new or unexpected safety concerns with JYNNEOS.16
- Most commonly reported adverse reactions to VAERS have been injection site symptoms (redness, swelling, pain, itching)16
- The frequencies of local and systemic reactions reported are similar to those reported in clinical trials16
- Myocarditis and pericarditis are adverse events of special interest. Observed rates are consistent with expected background rates16
- No reported cases of progressive vaccinia, eczema vaccinatum, or blindness18
- No recommended restrictions on blood or organ donation15
LIMITATIONS: Data based on voluntary reports through safety monitoring systems; not all potential reactions are captured. Estimations of frequency or establishment of causation may not be possible.
Dosing & Administration
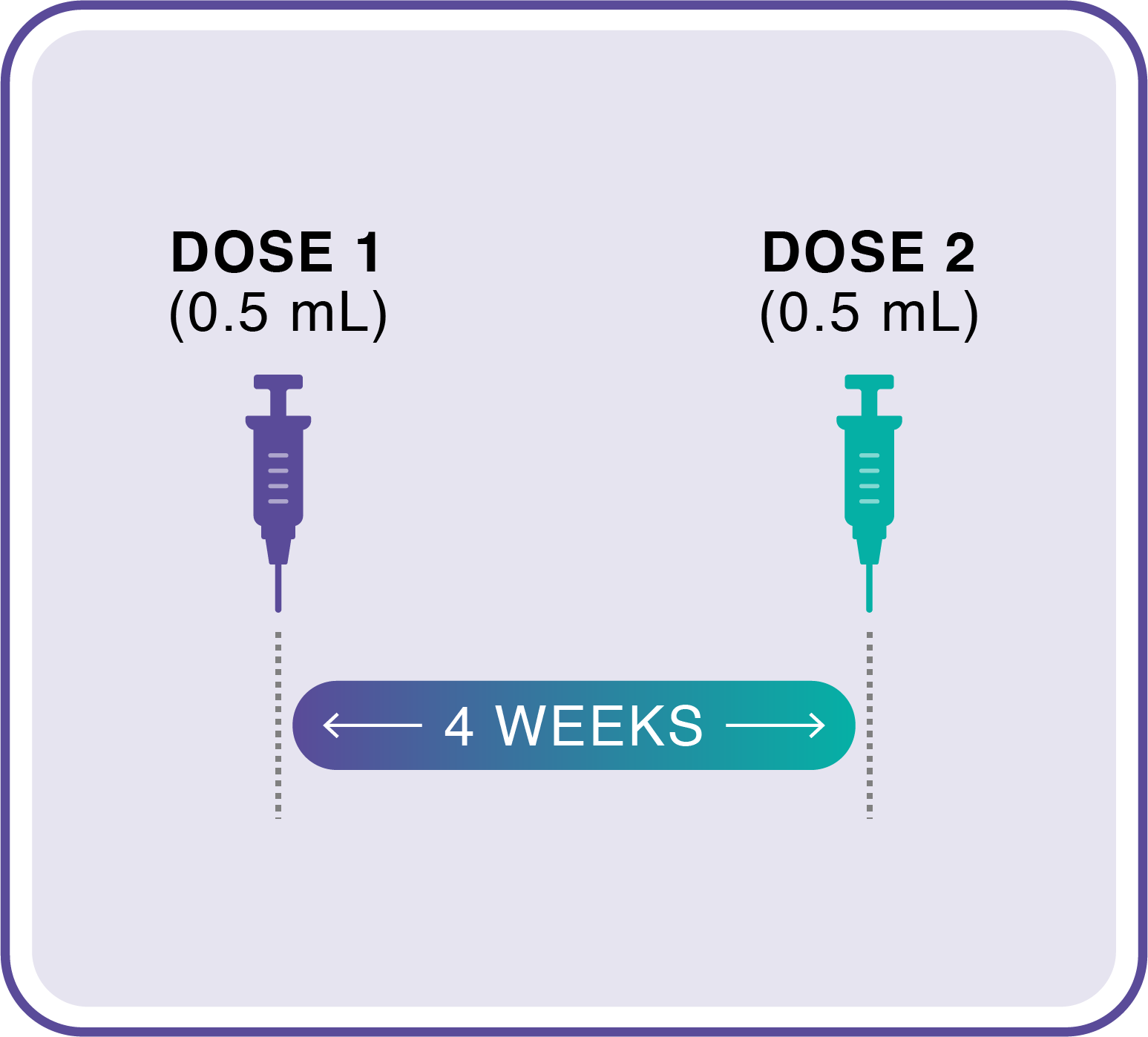
Help protect your appropriate patients against mpox in 2 shots
JYNNEOS is administered in 2 doses given 4 weeks apart.15
HOW SUPPLIED
PACKAGING information

*An FDA-assigned 10-digit NDC is converted by healthcare systems/payers to the HIPAA-standardized 11-digit NDC format by adding a leading zero to the applicable short segment. The HIPAA conversion for JYNNEOS is 50632-0001-03 and 50632-0001-02 (new zero underlined and highlighted). When submitting an insurance claim, it is generally recommended to use the NDC that is printed on the package of the medication. Please check with your insurance provider to confirm any specific requirements or preferences they may have regarding the submission of NDC information with your claim.
Storage conditions
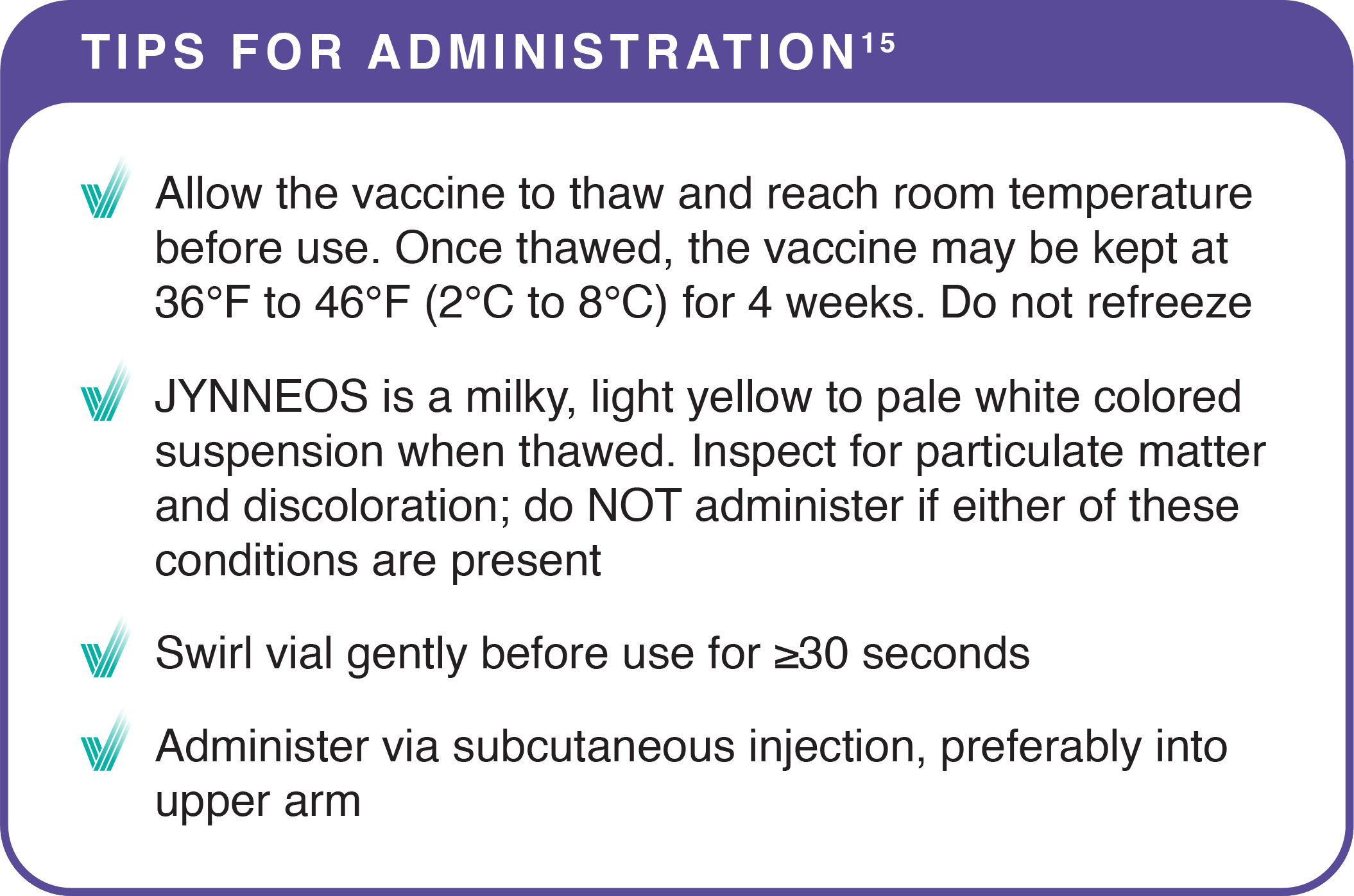
Store JYNNEOS frozen at -13°F to 5°F (-25°C to -15°C) and in the original package to protect from light. Once thawed, the vaccine may be kept at 36°F to 46°F (2°C to 8°C) for 4 weeks.15
Do not refreeze a vial once thawed. Do not use the vaccine after the expiration date shown on the vial label.15
For medical inquiries about JYNNEOS, please contact Medical Information at (844) 422-8274 or medical.information_na@bavarian-nordic.com.

Indication and Usage
JYNNEOS® is approved for the prevention of smallpox and monkeypox disease in adults 18 years of age and older determined to be at high risk for smallpox and monkeypox infection.
Important Safety Information
Appropriate medical treatment must be available to manage possible anaphylactic reactions following administration of JYNNEOS. Anyone who has who experienced a severe allergic reaction following a previous dose of JYNNEOS or following exposure to any component of JYNNEOS may be at increased risk for severe allergic reactions.
Syncope (fainting) has been reported following vaccination with JYNNEOS. Procedures should be in place to avoid injury from fainting.
Immunocompromised persons, including those receiving immunosuppressive therapy, may have a diminished immune response to JYNNEOS.
Vaccination with JYNNEOS may not protect all recipients.
In smallpox vaccine-naïve healthy adults, the most common (>10%) solicited injection site reactions were pain (84.9%), redness (60.8%), swelling (51.6%), induration (firmness at the injection site) (45.4%), and itching (43.1%); the most common solicited systemic adverse reactions were muscle pain (42.8%), headache (34.8%), fatigue (30.4%), nausea (17.3%) and chills (10.4%).
In healthy adults previously vaccinated with a smallpox vaccine, the most common (>10%) solicited injection site reactions were redness (80.9%), pain (79.5%), induration (70.4%), swelling (67.2%), and itching (32.0%); the most common solicited systemic adverse reactions were fatigue (33.5%), headache (27.6%), and muscle pain (21.5%).
The frequencies of solicited local and systemic adverse reactions among adults with HIV infection and adults with atopic dermatitis were generally similar to those observed in healthy adults.
Across all studies, a causal relationship to JYNNEOS could not be excluded for 5 SAEs, all non-fatal, which included Crohn’s disease, sarcoidosis, extraocular muscle paresis, throat tightness, and hemolytic anemia.
Among the cardiac AESIs reported, 6 cases (<0.1%) were considered to be causally related to JYNNEOS vaccination and included tachycardia, electrocardiogram T wave inversion, electrocardiogram abnormal, electrocardiogram ST segment elevation, electrocardiogram T wave abnormal, and palpitations. None of the cardiac AESIs considered causally related to study vaccination were considered serious.
JYNNEOS is for subcutaneous injection.
To report SUSPECTED ADVERSE REACTIONS, contact Bavarian Nordic at 1-833-365-9596 or the US Department of Health and Human Services by either visiting www.vaers.hhs.gov/reportevent.html or calling 1-800-822-7967.
ACIP, Advisory Committee on Immunization Practices; CDC, Centers for Disease Control and Prevention; FDA, US Food and Drug Administration; GMT, geometric mean titer; HHS, Health and Human Services; MPXV, monkeypox virus; NDC, National Drug Code; PRNT, Plaque Reduction Neutralization Test; VAERS; Vaccine Adverse Event Reporting System; WHO, World Health Organization.
References:
- CDC. JYNNEOS vaccine. Accessed January 10, 2024. https://www.cdc.gov/mpox/vaccines/index.html
- CDC. ACIP recommendations. Accessed January 10, 2024. https://www.cdc.gov/vaccines/acip/recommendations.html
- WHO. Fact sheets. Mpox (monkeypox). Accessed January 10, 2024. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Americo JL, et al. Proc Natl Acad Sci U S A. 2023;120(8):e2220415120.
- Mitjà O, et al. Lancet. 2023;401(10370):60-74.
- WHO. Multi-country outbreak of mpox, external situation report #31- 22 December 2023. Accessed March 6, 2024. https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report-31---22-december-2023
- CDC. 2022-2023 outbreak cases and data. Accessed January 10, 2024. https://www.cdc.gov/poxvirus/mpox/response/2022/index.html
- CDC. About mpox. Accessed January 10, 2024. https://www.cdc.gov/poxvirus/mpox/about/index.html
- Pollock ED, et al. MMWR Morb Mortal Wkly Rep. 2023;72:568–573.
- GOV.UK. Mpox (monkeypox): background information. Accessed March 7, 2024. https://www.gov.uk/guidance/monkeypox
- Owens LE, et al. MMWR Morb Mortal Wkly Rep. 2023;72:342–347.
- CDC. Mpox vaccine administration in the U.S. Accessed January 10, 2024. https://www.cdc.gov/poxvirus/mpox/response/2022/vaccines_data.html
- McQuiston JH, et al. MMWR Morb Mortal Wkly Rep. 2023;72:547–552.
- CDC. CDC changes monkeypox terminology to mpox. Accessed January 10, 2024. https://www.cdc.gov/nchhstp/dear_colleague/2022/dcl-changes-monkeypox-terminology.html
- JYNNEOS Prescribing Information. Bavarian Nordic; 2023.
- CDC. 2022/2023 Mpox outbreak: situational awareness and updates (October 25, 2023, ACIP meeting presentation). Accessed January 10, 2024. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-10-25-26/02-MPOX-Minhaj-508.pdf
- CDC. JYNNEOS vaccine effectiveness (February 22, 2023, ACIP meeting presentation). Accessed January 10, 2024. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-22/mpox-04-chard-508.pdf
- CDC. CDC WONDER. Accessed February 23, 2024. https://wonder.cdc.gov/vaers.html
Indication and Usage
JYNNEOS® is approved for the prevention of smallpox and monkeypox disease in adults 18 years of age and older determined to be at high risk for smallpox and monkeypox infection.
Important Safety Information
Appropriate medical treatment must be available to manage possible anaphylactic reactions following administration of JYNNEOS. Anyone who has who experienced a severe allergic reaction following a previous dose of JYNNEOS or following exposure to any component of JYNNEOS may be at increased risk for severe allergic reactions.
Syncope (fainting) has been reported following vaccination with JYNNEOS. Procedures should be in place to avoid injury from fainting.
Immunocompromised persons, including those receiving immunosuppressive therapy, may have a diminished immune response to JYNNEOS.
Vaccination with JYNNEOS may not protect all recipients.
In smallpox vaccine-naïve healthy adults, the most common (>10%) solicited injection site reactions were pain (84.9%), redness (60.8%), swelling (51.6%), induration (firmness at the injection site) (45.4%), and itching (43.1%); the most common solicited systemic adverse reactions were muscle pain (42.8%), headache (34.8%), fatigue (30.4%), nausea (17.3%) and chills (10.4%).
In healthy adults previously vaccinated with a smallpox vaccine, the most common (>10%) solicited injection site reactions were redness (80.9%), pain (79.5%), induration (70.4%), swelling (67.2%), and itching (32.0%); the most common solicited systemic adverse reactions were fatigue (33.5%), headache (27.6%), and muscle pain (21.5%).
The frequencies of solicited local and systemic adverse reactions among adults with HIV infection and adults with atopic dermatitis were generally similar to those observed in healthy adults.
Across all studies, a causal relationship to JYNNEOS could not be excluded for 5 SAEs, all non-fatal, which included Crohn’s disease, sarcoidosis, extraocular muscle paresis, throat tightness, and hemolytic anemia.
Among the cardiac AESIs reported, 6 cases (<0.1%) were considered to be causally related to JYNNEOS vaccination and included tachycardia, electrocardiogram T wave inversion, electrocardiogram abnormal, electrocardiogram ST segment elevation, electrocardiogram T wave abnormal, and palpitations. None of the cardiac AESIs considered causally related to study vaccination were considered serious.
JYNNEOS is for subcutaneous injection.
To report SUSPECTED ADVERSE REACTIONS, contact Bavarian Nordic at 1-833-365-9596 or the US Department of Health and Human Services by either visiting www.vaers.hhs.gov/reportevent.html or calling 1-800-822-7967.
These Terms and Conditions govern the VIVOTIF® Dose Replacement Program (the “Program”) operated by Emergent Travel Health (“Emergent”).
Program Participation Eligibility:
The Program will be available, subject to these Terms and Conditions, to health care practitioners (“Program Participants”) who (i) have purchased VIVOTIF either directly from Emergent or from a third party who purchased the product from Emergent, (ii) have patients who have improperly administered and/or stored VIVOTIF (as determined by Emergent in its sole discretion) and who the Program Participants have directed to restart taking VIVOTIF and (iii) comply with these Terms and Conditions. Emergent has the sole authority to determine eligibility for participation in the Program.
Product Eligibility:
The Program is available for doses of VIVOTIF which have been improperly administered or stored by patients of Program Participants so long as the patients have been directed by the Program Participant to restart taking VIVOTIF. In order to receive credit or replacement product for VIVOTIF, Program Participants must contact Emergent Medical Information at medicalinformation@ebsi.com or 1-800-533-5899 to determine the viability of the improperly administered or stored doses of VIVOTIF.
For the avoidance of doubt, the Program is not available for, and no credit or replacement product will be issued in respect of, (i) any product for which a patient has not been directed to restart taking VIVOTIF, (ii) any lost or misplaced product or any product for which a patient has experienced an adverse reaction or side effect but which has not been improperly administered or stored, (iii) any product (whether administered or unused) for which reimbursement has been or will be requested or collected or (iv) any product damaged as a result of unforeseen power outages, natural disasters or other emergency situations.
Program Participants may request credit or replacement product for VIVOTIF satisfying the criteria set forth herein for up to five units of VIVOTIF (each unit contains four doses) during each calendar year. Emergent has the sole authority to determine product eligibility for the Program.
Enrollment and Requests for Credit or Replacement Product:
Program Participants are required to enroll in the Program and request a credit or product replacement from Emergent by fully and accurately completing the Program Administration Form available on the Product Replacement Program section of our site and by submitting the completed and signed form to Emergent Customer Care via facsimile at 800-392-9490, e-mail at customercare@ebsi.com, or online at the Product Replacement Program section of our site.
Issuance of Credit or Product Replacement:
Emergent will determine, in its sole discretion, whether any submitted request complies with these Terms and Conditions, whether to accept or reject any submitted request and whether to issue a credit or product replacement. Credits will only be available for Program Participants who have purchased VIVOTIF directly from Emergent. For all other Program Participants, only product replacement will be available under the Program. In the event Emergent elects to accept a request and issue a credit, such credit will be equal to the lesser of (i) the net purchase price paid to Emergent by the Program Participant for the doses of VIVOTIF described in the Program Administration Form and (ii) the purchase price established by Emergent for VIVOTIF purchases by the Program Participant and in effect as of the date of the credit request. Such credit will apply solely to future purchases of VIVOTIF or other products from Emergent. In the event Emergent elects to accept a request and provide replacement product, it will deliver the replacement product to the Program Participant (at Emergent’s cost and expense).
Recordkeeping:
Each Program Participant will be obligated to maintain accurate books and records supporting any credit or product replacement request made under the Program for a period of five years following the later of (i) the date of the last request submitted hereunder by such Program Participant and (ii) the termination date of the Program. Emergent or its agent may, during normal working hours and with reasonable advance notice, inspect, audit and reproduce such books and records (x) to validate any request made hereunder and/or any representation made on any Program Administration Form by such Program Participant, or (y) as otherwise requested by governmental authorities or required by applicable law or regulation.
Warranty and Disclaimer:
TO THE MAXIMUM EXTEND PERMITTED BY LAW, EMERGENT PROVIDES THE PROGRAM “AS IS” WITH NO WARRANTIES OF ANY KIND AND EXPRESSLY DISCLAIMS ANY WARRANTY, EXPRESS OR IMPLIED, REGARDING THE PROGRAM, INCLUDING ANY IMPLIED WARRANTY OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE OR NON-INFRINGEMENT.
Limitation of Liability:
UNDER NO CIRCUMSTANCES WILL EMERGENT OR ITS AFFILIATES OR THEIR RESPECTIVE SHAREHOLDERS, AGENTS, EMPLOYEES, MANAGERS, OFFICERS OR DIRECTORS BE LIABLE FOR ANY INDIRECT, INCIDENTAL, CONSEQUENTIAL, SPECIAL, PUNITIVE OR EXEMPLARY DAMAGES ARISING OUT OF OR IN CONNECTION WITH PARTICIPATION IN THE PROGRAM, WHETHER OR NOT Emergent HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES.
Sole Remedy:
Any Program Participant who is dissatisfied with the Program or these Terms and Conditions will have as the sole and exclusive remedy the discontinuation of participation in the Program.
Indemnification:
Each Program Participant agrees to indemnify, defend and hold harmless Emergent and its affiliates and their respective shareholders, agents, employees, managers, officers and directors from and against any and all claims, actions, causes of action, liabilities, losses and expenses (including reasonable attorneys’ fees) arising from (i) the Program Participant’s participation in the Program, (ii) the Program Participant’s violation of these Terms and Conditions and (iii) any violation or failure by the Program Participant to comply with all applicable laws and regulations relating to the Program.
Termination:
Emergent reserves the right to terminate or suspend the Program or to remove a Program Participant from the Program, in each case for any reason without notice in its sole discretion. No requests for credit or product replacement will be accepted after termination of the Program or removal of a Program Participant from the Program, regardless of whether the applicable VIVOTIF purchase or request was made prior to such termination or removal.
Amendments:
Emergent reserves the right to amend these Terms and Conditions at any time in its sole discretion.
Governing Law:
These Terms and Conditions will be governed by the laws of the State of Delaware without regard to conflicts of laws principles. Any dispute arising hereunder will be subject to final and binding arbitration in San Mateo County, California before the American Arbitration Association under its then applicable rules for commercial disputes. Each Program Participant and Emergent waive any rights to have any dispute resolved in a court of law by a judge or jury.
Interpretation:
Emergent has the sole authority to interpret these Terms and Conditions and to determine eligibility for participation in the Program and compliance with these Terms and Conditions.
Program Assistance:
For questions or additional information regarding the Program, please visit the Product Replacement Program section of our site or contact Emergent Customer Care at 1-800-533-5899.



